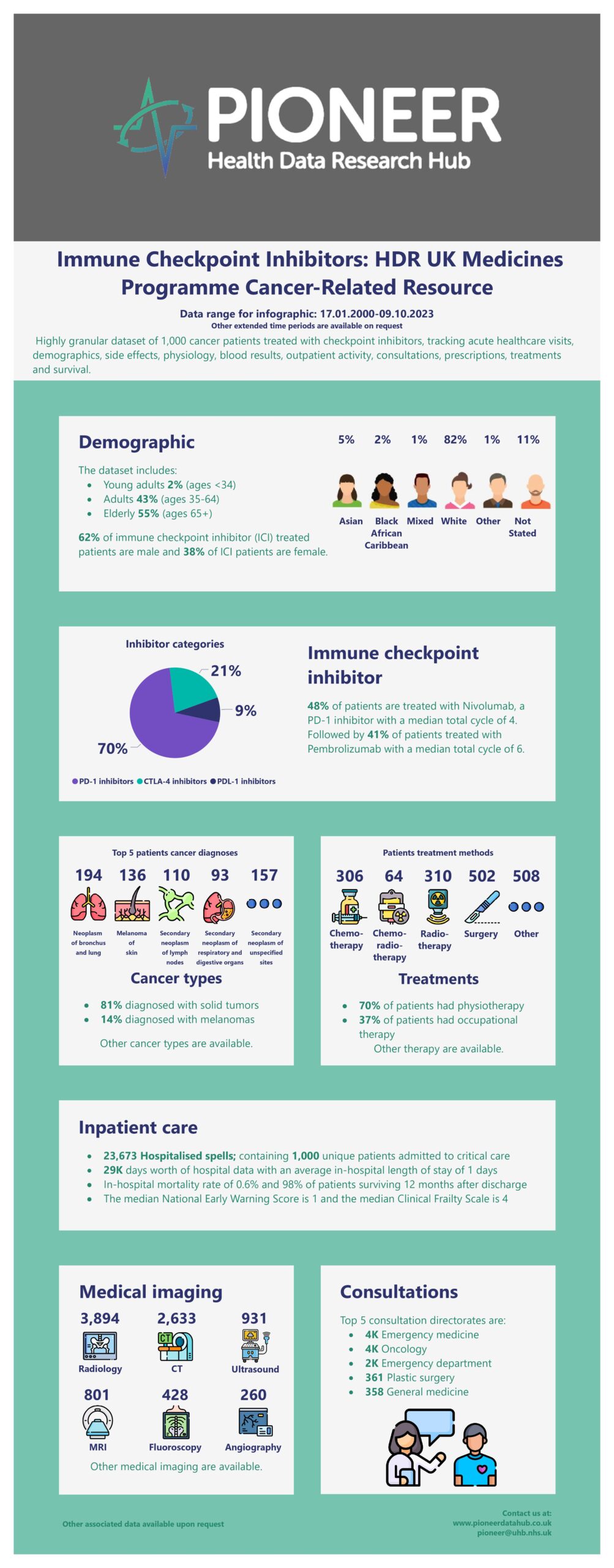
A highly granular, medicines-focused dataset of approximately 1,000 patients over 3 years. Includes patient demographics & co-morbidities taken from ICD-10 & SNOMED-CT codes. Serial, structured data pertaining to acute care process (timings, readmissions, survival), primary diagnosis, presenting complaint, physiology readings (pulse, blood pressure, respiratory rate, oxygen saturations and others), extensive blood results (infection, inflammatory markers) and acuity markers such as AVPU Scale, NEWS2 score, SEWS score, imaging reports, consultation, therapy, referrals, complete documentation of all prescribed & administered treatments including fluids, blood products, procedures, information on outpatient admissions and survival outcomes following one year post discharge.
This medicines-focused dataset is an invaluable resource for researchers aiming to analyse and compare the effects of checkpoint inhibitors on patients. It offers an opportunity to understand treatment pathways and healthcare utilisation in this specific patient cohort. Dive into this rich data source to uncover new insights and contribute to the evolving field of cancer immunotherapy.
Geography: The West Midlands (WM) has a population of 6 million & includes a diverse ethnic & socio-economic mix. UHB is one of the largest NHS Trusts in England, providing direct acute services & specialist care across four hospital sites, with 2.2 million patient episodes per year, 2750 beds & > 120 ITU bed capacity. UHB runs a fully electronic healthcare record (EHR) (PICS; Birmingham Systems), a shared primary & secondary care record (Your Care Connected) & a patient portal “My Health”.
Data set availability: Data access is available via the PIONEER Hub for projects which will benefit the public or patients. This can be by developing a new understanding of disease, by providing insights into how to improve care, or by developing new models, tools, treatments, or care processes. Data access can be provided to NHS, academic, commercial, policy and third sector organisations. Applications from SMEs are welcome. There is a single data access process, with public oversight provided by our public review committee, the Data Trust Committee. Contact [email protected] or visit www.pioneerdatahub.co.uk for more details.
Available supplementary data: Matched controls; ambulance and community data. Unstructured data (images). We can provide the dataset in OMOP and other common data models and can build synthetic data to meet bespoke requirements.
Available supplementary support: Analytics, model build, validation & refinement; A.I. support. Data partner support for ETL (extract, transform & load) processes. Bespoke and “off the shelf” Trusted Research Environment (TRE) build and run. Consultancy with clinical, patient & end-user and purchaser access/ support. Support for regulatory requirements. Cohort discovery. Data-driven trials and “fast screen” services to assess population size.
Further information including technical details, coverage, format and standards, provenance and related resources can be found on the link below: https://web.www.healthdatagateway.org/dataset/832b9428-da74-4bed-b35f-5a797bafd67e





